Research
We are witnessing the beginning of a new generation of medicine. The unprecedented amount of large-scale next-generation sequencing data from patients with cancer and other diseases makes data science and artificial intelligence (AI) play a pivotal role in biomedical research. The pressing question is how to best translate the accumulated data into a more effective practice of medicine. This question forms the cornerstone of our research articles.
1. Synthetic Lethality-based Precision Cancer Medicine
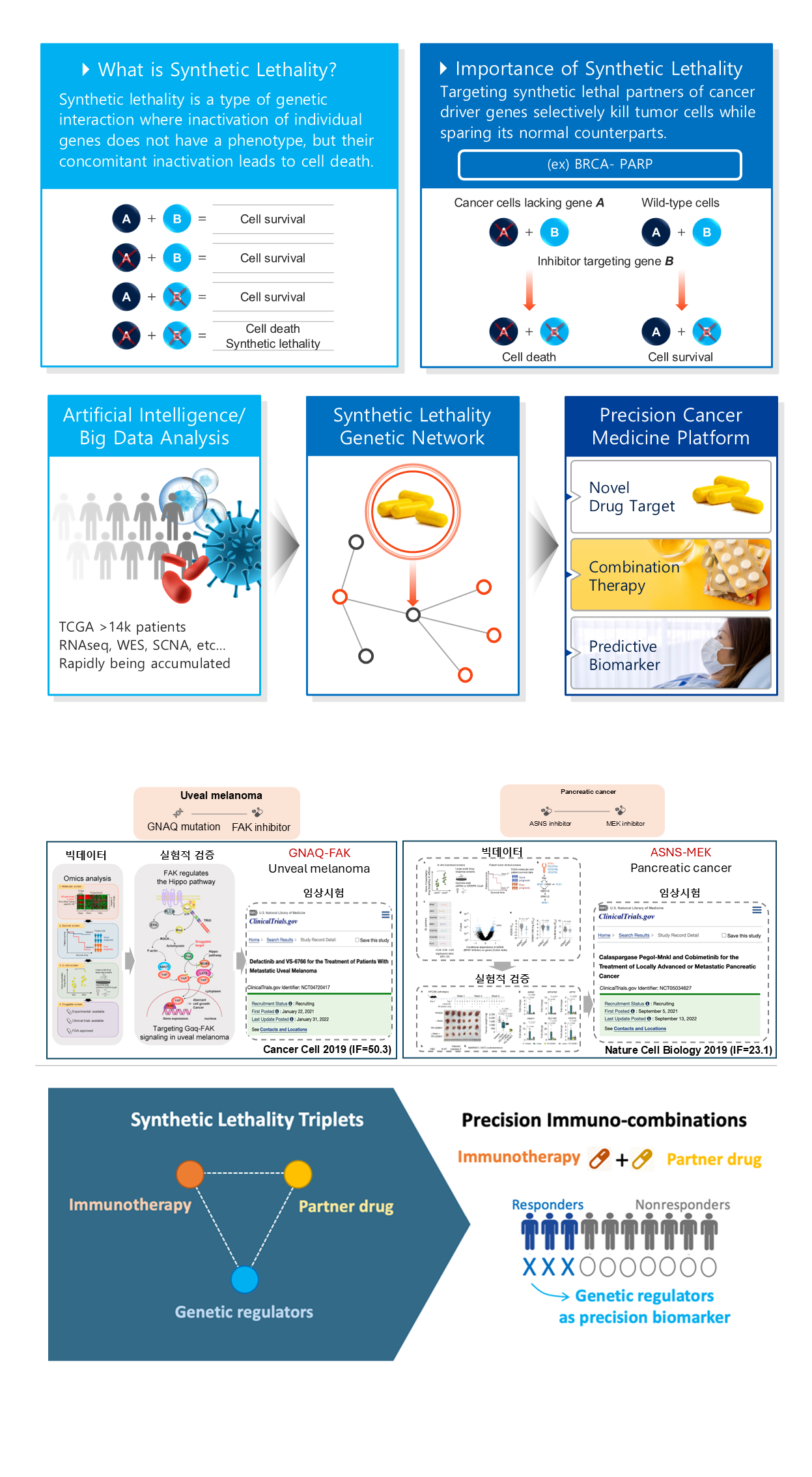
Joo has led pioneering studies based on the novel concept of synthetic Lethality. It describes the interaction between two genes: disabling one gene alone has little effect, but disabling both simultaneously causes cell death. Joo’s works focused on discovering drug targets with real clinical benefits using the concept of synthetic lethality (Feng et al. Cancer Cell 2019; Keshet, Lee et al. Nature Cancer 2020), advancing immunotherapy (Lee et al. Cell 2018; Lee et al. JAMA Oncology 2019), and realizing precision oncology (Lee et al. Nature Communications 2018; Lee et al. Cell 2021). In particular, the 2021 Cell paper was the first to demonstrate that synthetic lethality could enable precision medicine by matching cancer patients to the most effective drugs based on their genetic profiles.
Samsung Foundation Grant: Introducing “Triple Synthetic Lethality” (Link)
Harnessing the immune system to treat cancer is an appealing idea, since it represents the most natural way of healing. However, the majority of patients still do not benefit from this treatment. Therefore, finding combination therapies that can enhance the effectiveness of ICT has become a million-dollar question driving the global cancer drug market. Building on the previous work, Joo’s lab aims to establish a new concept: triple synthetic lethality. This refers to interactions among three genes, where cells only die if all three are inactivated. Using this concept, the research will:
- Provide a rational basis for selecting drugs for immunotherapy combinations.
- Identify biomarkers that can help select the right patient groups.
The discovery of new immunotherapy combinations through this approach will not only establish a paradigm of research and development driven by AI and big data but also secure key technologies that could lead the global immuno-oncology market.
2. From Slides to Spatial Omics: Toward Pathology-Based Precision Cancer Medicine
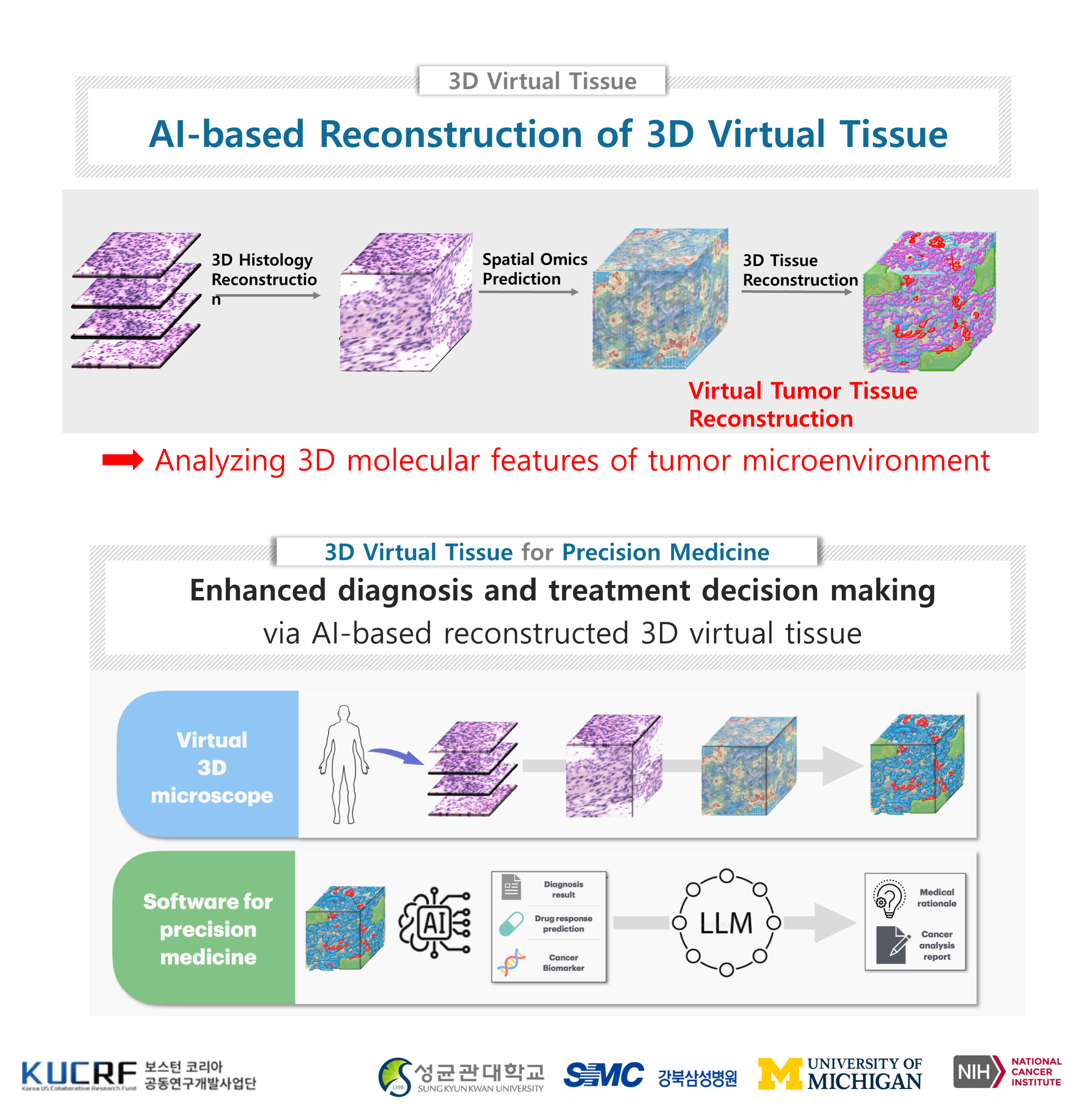
Cancer diagnosis has traditionally relied on looking at tissue samples under a microscope—what pathologists call “slides.” Scientists can now combine traditional pathology with spatial omics—technologies that map where genes and proteins are active directly within the tissue. Think of it as adding a molecular “Google Maps” layer on top of the slide, showing not just the shape of the cells but also the biological activity behind the shape. By integrating these layers of information, we can better understand how cancer cells interact with their surrounding environment. This could help answer questions like: Why does one patient respond to cancer therapy while another does not? And how can treatment be tailored to each individual’s cancer?
BostonKorea Grant: 3D Reconstruction of Tumor Microenvironment Characterization (Link)
We have been developing AI models to robustly predict spatial omics data and extend it from 2D to 3D. Imagine we are building a virtual tissue that resembles the tumor tissue given a few histopathology slides. With the virtual 3D tissue sections, we foresee that we can dramatically improve the diagnosis and treatment outcome prediction of cancer patients. The ultimate goal is precision cancer medicine—using detailed maps of tumors, both structural and molecular, to design the right treatment for the right patient at the right time. What started as a pathologist’s slide under a microscope is now evolving into a powerful multi-dimensional view of cancer that may transform care in the years ahead.
Funding
Our work is made possible by funding from the following organizations.






